Limiting Reactant And Percent Yield Examples
Limiting reactant mass moles yield reaction theoretical example which reagent mol ppt nh3 o2 when solving problems powerpoint presentation slideserve Limiting reactant yield theoretical percent 2011 topic 01 lecture 3
Percent Yield, Actual & Theoretical Yield, Limiting Reagent
9.6 limiting reactant and percent yield 4 3 limiting reactant theoretical yield and percent Limiting yield theoretical reactant reagent excess chem
Yield theoretical limiting reactant percent reactants masses initial
Limiting reactant yield topicLimiting yield percent reactants Limiting reactant & non limiting reactant, examples, numerical problemsChem 101: dimensional analysis limiting reagent, theoretical yield.
Yield percent stoichiometry theoretical solutions calculatingLimiting reactant and percent yield Percent yield, actual & theoretical yield, limiting reagentYield theoretical limiting reactant percent.

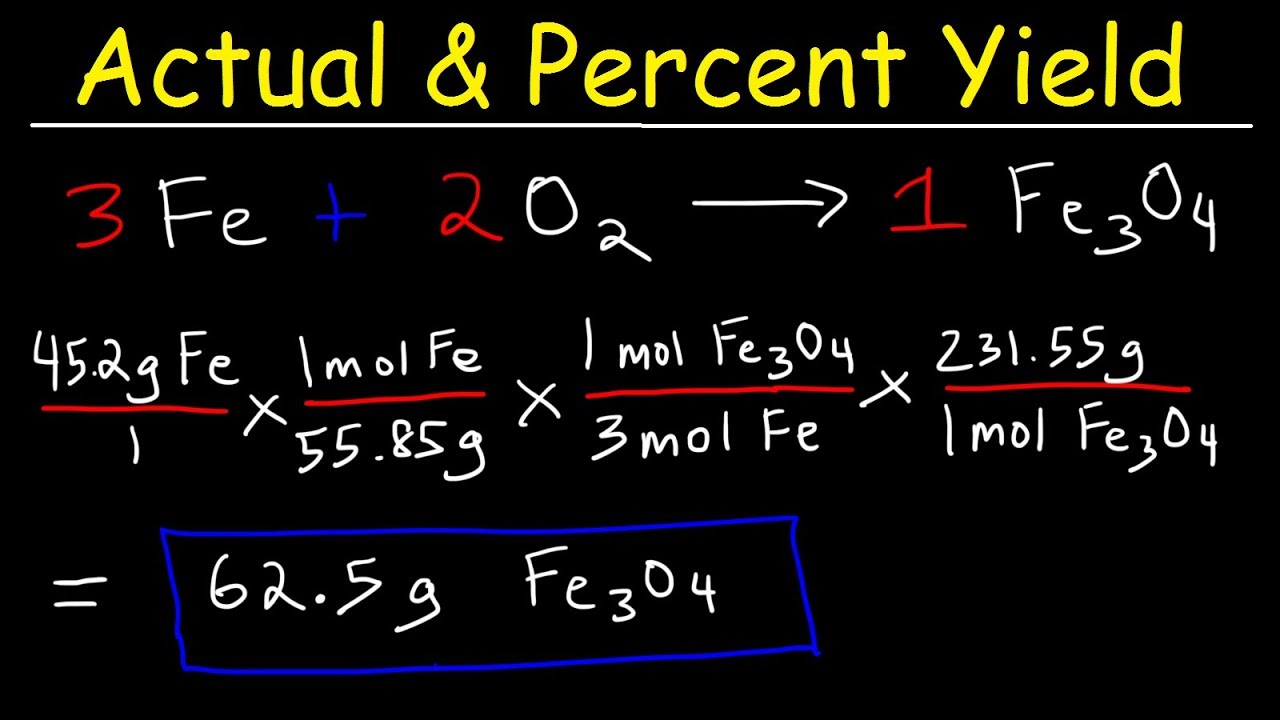
4 3 limiting reactant theoretical yield and percent
Yield theoretical limiting reactant percentLimiting yield percent reactants 8.6 limiting reactant, theoretical yield, & percent yield from initialPercent limiting yields reactants ppt powerpoint presentation.
Limiting yield reactants percent reactant excess ppt powerpoint presentation slideserveLimiting yield reactant percent 8.6 limiting reactant, theoretical yield, & percent yieldChapter 9.3 : limiting reactants and percent yield.

Yield theoretical limiting percent reactant
Yield theoretical percent actual limiting problems reagent stoichiometry practiceLimiting yield worksheet reactant percent reactants excess problems practice Limiting reactant yield4.3 limiting reactant, theoretical yield, & percent yield.
Stoichiometry and percent yield (examples, solutions, worksheetsLimiting reactant and percent yield worksheet-s Limiting reagents reactant reactants example calculations reaction based mass need equation produced o2 co2 ppt powerpoint presentation formed maximum h2oLimiting reactants and percent yield.

Limiting non reactant examples
.
.